Low FDA Approvals of New Biopharmaceuticals Continued in 2008
Low FDA Approvals of New Biopharmaceuticals Continued in 2008
Background - This article updates the FDA Approvals - Review 1981-2007, Editorial and Listings from 2002-Present Web page and the associated article, "Paucity of Biopharma Approvals Raises Alarm: Lower Numbers, Novelty, and Economic Impact Indicate Problems," published in Genetic Engineering News. See these for further discussions of long-term trends. See also a running list of recent years' approvals; and check out BIOPHARMA: Biopharmaceutical Products in the U.S. and European Markets, the only reference/information resource (database) concerning biopharmaceuticals -- the free public databases may be used to retrieve products by year of approval and many other characteristics. [BIOPHARMA currently (1/2009) provides 318 monographs for currently FDA-approved biopharmaceuticals (a few considered borderline), including 117 for recombinant proteins/Mabs].
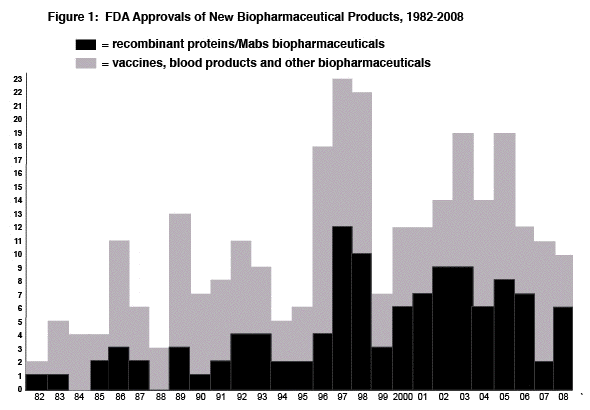
Figure 1 graphs the number of approvals of new biopharmaceutical products by year since 1982 (when the first recombinant product was approved).
*Approvals involving simple mixtures or combination use of previously approved products; not considered to be new biopharmaceutical products | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The low numbers of biopharmaceutical approvals in recent years are of particular concern, because a large number of products have been in the development pipeline. BIOPHARMA reports over 25 product applications currently pending with FDA and over 25 applications expected to be filed in 2009. As many have noted, in recent years, an increasing number of products have been experiencing delays in filing, FDA evaluation and/or their development has been abandoned very late in development, including after Phase III trals and even after filing for approval. Complete response letters and other reports of problems with filings are now common. However, unless the great majority of pending and upcoming applications are rejected, delayed or abandoned, significant increases in approvals can be expected in 2009 and coming years.
There were only 13 full approvals (BLAs, NDAs) for biopharmaceutical products in 2008. In the decade 1996-2005, there was an average of 16.6 new product approvals/year. Eight of the 13 full approvals in 2008 (involving 6 new products) involved recombinant proteins, up from only 2 in 2007, returning to a level more typical for approvals since 2000. However, full approval does not mean that a product is actually new. In recent years, full approvals (seemingly more and with no discernable consistency) have been granted to marketed products for new indications, regimens and manufacturing changes -- changes traditionally handled by supplemental approvals.
Multiple 2008 approvals involved already-approved products, with no little or nothing new with the product, its active agent or manufacture. Three 2008 approvals were for mixtures or combination regimens using products already on the market: 1) Novolog Mix 50/50 (a mixture of regular modified recombinant insulins, with both components and a 70/30 mix already marketed); 2) Pentacel (a 4-antigen combination vaccine mixed with a monovalent vaccine prior to injection); and 3) PegPak (combined use, already well-established, and packaging of recombinant pegylated alpha-interferon with the drug ribavirin). Although these are new full approvals, these are not considered or counted as being new products. Eliminating these trivial approvals, 2008 had only 10 approvals of products that the author considers Novel Biopharmaceutical Entities (NBEs), i.e., products with substantially novel active agents, manufacturing processes and/or formulations. This is the lowest number since 1999. Further, although considered a new and unique/distinct product due to substantive changes in formulation and manufacture, Xyntha (recombinant Factor VIII) from Bayer is essentially an updated version of ReFacto, with its active agent unchanged. And Accretropin, which received 505(b)(2) generic drug approval, by definition, lacks considerable novelty. [Approved products are each further discussed below].
The number of such full vs. the usual supplemental approvals granted to already-marketed biopharmaceuticals for new indications and incremental changes in manufacture or formulation has been increasing in recent years. Potential reasons for this include manufacturers seeking to game the system in the future by filing and obtaining full vs. supplemental BLAs in expectation of retrospective (grandfathered) years of marketing exclusivity that may be granted to "new" (based on receiving BLA approval) biologics with future implementation of biosimilar/follow-on biologics regulations (see www.biosimilarspipeline.com); the FDA desperately seeking to boost the dismal number of new biophamaceutical approvals; and/or FDA seeking increased user fees from processing full vs. supplemental approvals.
Two 2008 products received abbreviated 505(b)(2) generic drug approvals for what some might now consider to be follow-on proteins or biosimilars -- Accretropin (recombinant human growth hormone) and Novolog Mix 50/50. Despite receiving approval as a generic drug based largely on comparisons with an already-approved product, Accretropin, itself, is considered new and unique (e.g., it is not interchangeable with its reference product). This is unlike Novolog Mix 50/50, which is a mixture of two approved recombinant insulins, with a 70/30 mix already marketed, which is not considered a truly new or novel product.
Advances and Innovations Were Mostly Marginal or Incremental
Hardly any monoclonal antibodies and cancer therapeutics have been approved in recent years, despite many reports (much hype) about large numbers in late development and claims that these products have revolutionized (or will) patient care. Only one monoclonal antibody product received approval in 2008 (Cimzia), 2007 and 2006. If one also wants to consider Nplate, containing a recombinant peptibody, and Araclyst, containing a recombinant trap molecule, with both active agents designed to have similarities with monoclonal antibodies, then three monoclonal antibodies received approval in 2008. The large number of chimeric, humanized and fully human monoclonal antibodies and cancer therapeutics in development that many companies and much of the industry are relying on for new products and continued survival has yet to materialize in the form of marketed products. The situation is more desolate with cancer therapeutics. No biopharmaceuticals were approved for cancer indications in either 2008 or 2007, with only one approved in 2006.
Products approved in 2008 expected to provide the most significant advances in patient care are Arcalyst, the first treatment for Cryopyrin-Associated Periodic Syndromes (CAPS) disorders (orphan diseases); Cinryze, the first treatment for hereditary angioedema (HAE; an orphan disease); and NPLATE, providing a new alternative for treatment of refractory immune thrombocytopenic purpura (ITP). The other products largely involve incremental advances (of varying significance) and approvals for indications for which treatments and often similar products are already available, and in this respect may be considered 'me too" type products to varying extents.
In terms of economic impact, no 2008-approved products are expected to achieve blockbuster sales or sales over $1 billion/year (for their currently-approved indications), but sales of 2008-approved products will be much greater than those approved in 2007. Total sales for all 2008 products will easily peak at over one or several $billion/year, unlike 2007-approved products, which combined were not expected to attain blockbuster-level sales.
There was a significant increase in the number of approvals (7) in 2008 involving U.S.-based companies (Lev, Amgen, Schering-Plough, Baxter, Regeneron, Wyeth and Zymogenetics). This contrasts with 2007, when only one approval involved a U.S. biotech-type company (Genzyme; an approval for a product already on the market for decades). Among 2008 products, those developed and marketed by smaller U.S. biotech-type companies include Arcalyst from Regeneron and Recothrom from Zymogenetics, with one product marketed (not developed) in the U.S. by Lev Pharmaceuticals. Among companies with products approved in 2008, Schering-Plough, GlaxoSmithKine, Baxter, Bayer, Novo Nordisk, Sanofi Pasteur (Sanofi Aventis) and Wyeth are large international pharmaceutical companies, and depending on criteria, Amgen may now be considered to be included in this class. A number of 2008 approvals (6) involved European-based companies (Novo Nordisk, UCB, Sanofi Pasteur and Bayer and Sanquin), and one product involved a Canadian company (Cangene and Apotex).
In terms of technology, particularly genetic engineering, 2008 offered increased innovation, including two engineered monoclonal antibody-like agents. The active agent in Nplate from Amgen is an E. coli-expressed "peptibody" or
recombinant Fc-peptide fusion protein with antibody-like binding affinity for a novel target, thromobopoietin. Arcalyst from Regeneron contains IL-1 Trap (rilonacept), a Chinese hamster ovary (CHO) cell-expressed recombinant dimeric fusion single-chain antibody-like glycoprotein consisting of two human IL-1 cytokine receptor extracellular domains targeting IL-1 (a novel target) linked in-line to the Fc (constant) portion of human immunogloblulin G1 (IgG1). Cimzia, developed by Celltech, now UCB Group, contains an E. coli-expressed recombinant Fab' portion of a recombinant humanized tumor necrosis factor (TNF) murine (mouse) monoclonal antibody with specificity for tumor necrosis factor-alpha (joining other products with this target). Recothrom from Zymogenetics is the first recombinant human thrombin product. Xyntha from Wyeth contains the same modified recombinant Factor VIII as in ReFacto, which it is replacing, but the product's manufacture has been significantly updated, with all use of animal- and human-derived products eliminated, including use in culture media and the replacement of a monoclonal antibody used for immunoaffinity purification with a synthetic ligand. Other products offered, at best, incremental advances in active agent design and product manufacture. All of the recombinant products approved in 2008 involved use of conventional (the same old) expression systems.
Review of the 2008-Approved Products
1) Cinryze, human plasma-derived C1-esterase inhibitor from Sanquin, with U.S. marketing by Lev Pharmaceuticals, recently acquired by ViroPharma, was approved for prophylaxis against attacks of hereditary angioedema (HAE) or C1 inhibitor deficiency. Cinryze is the first C1 inhibitor product approved for treatment of HAE in the U.S. HAE is a rare disease and Cinryze has orphan designation. ViroPharma projects peak sales around $250 million/year.
2) Novolog Mix 50/50 from Novo Nordisk is a mixture of recombinant insulin aspart (NovoLog), human insulin with a modified sequence, and regular human insulin used for treatment of diabetes. Containing already-approved component products and similar to already-approved 70/30 mix, Novolong Mix received an abbreviated 505(b)(2) generic drug approval, is not considered a new product, and could be considered a follow-on protein- or biosimilar-type approval.
3) NPLATE, a recombinant thrombopoietin antibody-like "peptibody," from Amgen received approval for treatment of adults with chronic immune thrombocytopenic purpura (ITP). This provides a new option for ITP patients not responding to immune suppression or surgery. Sales are projected to peak at $300-$500 million/year.
4) Pentacel from Sanofi Pasteur is a combination vaccine involving use of ActHIB (Haemophilus influenza vaccine) to reconstitute (dilute) Quadracel, a lyophilized quadravelent vaccine containing diphtheria, tetanus, acellular pertussis and inactivated polio vaccine components. Both ActHIB and Quadracel were already on the U.S. market, and this is considered as essentially a combination use regimen (or virtual product), rather than a new, unique/distinct product. Pentacel will compete with combinations of other combination and monovalent vaccines.
5) PegPak from Schering-Plough involves the combined use, already well-established, and packaging of recombinant pegylated alpha-interferon (PEG-Intron) with the drug ribavirin (Rebetol) for treatment of chronic hepatitis C, with both of these products already marketed. If considered as a product (combining the 2 products' sales), PegPak already has acheived blockbuster status, with >$700 million in worldwide sales of PEG-Intron in 2007 and Rebetol sales estimated at >$300 million.
6) Cimzia, a recombinant tumor necrosis factor monoclonal antibody Fab fragment--polyethylene glycol (PEG) polymer conjugate, from UCB, with U.S. marketing by Bayer Schering, received approval for treatment of refractory Crohn's disease in adults. Unlike Remicade, another TNF inhibitor approved for Crohn's disease, Cinzia is administered by subcutaneous injection, while Remicade is administered by intravenous infusion. With approvals expected for other indications with larger markets, e.g,. rheumatoid arthritis, Cimzia has potential to become a blockbuster (>$1 billion/year sales).
7) Rotarix, live oral rotavirus vaccine, from GlaxoSmithKline (GSK), with early development by AVANT, now Celldex Therapeutics, received approval for prevention of rotavirus gastroenteritis in infants, becoming the second rotavirus vaccine available in the U.S. Rotarix contains a single live human reassortant (natural recombinant) rotavirus, while the other vaccine, RotaTeq from Merck, contains five live bovine/human reassortant (natural recombinant) rotavirus strains. Peak sales of Rotarix are projected to be $500 million or more.
8) Kinrix, a quadravalent (4-antigen) vaccine formulation containing diphtheria, tetanus, acellular pertussis, and inactivated polio vaccine components (each previously approved alone and/or in other combination vaccines), from GlaxoSmithKline (GSK) received approval, but only as a fourth or fifth dose booster vaccine in infants having received prior vaccination with its various component vaccines. Although containing already-approved components, this combination/formulation is novel enough (marginally) to be considered to be a new, unique/distinct product.
9) Artiss, a fibrin sealant kit, from Baxter involves human plasma-derived fibrinogen and thrombin that are mixed and used to glue skin grafts in place. This joins other fibrin glue kits, although Artiss is the only one specifically approved for skin grafts.
10) Arcalyst, recombinant IL-1 Trap, a recombinant dimeric fusion single-chain antibody-like glycoprotein involving two linked human IL-1 cytokine receptor domains, from Regeneron received approval for long term treatment of two Cryopyrin-Associated Periodic Syndromes (CAPS) disorders: Familial Cold Auto-Inflammatory Syndrome (FCAS) and Muckle-Wells Syndrome (MWS). Previously, no specific therapeutics were available for these orphan diseases.
11) Xyntha, recombinant b-domain deleted Factor VIII, from Wyeth is essentially an updated version of ReFacto, which it is replacing. Xyntha's updated manufacture and formulation provides decreased theoretical risk of prion and viral infection. FDA recognizes that both Xyntha and previous ReFacto contain the same active agent, with Xyntha having modified manufacture and formulation eliminating the use of human- or animal-derived products, including use of a synthetic ligand replacing a monoclonal antibody for immunoaffinity purification. Although receiving a full BLA (with FDA converting the sBLA filed by Wyeth to a full BLA late in the product's evaluation), with no change in its active agent or use, Xyntha could be considered a variation or new version of ReFacto, with such manufacturing and formulation updates traditionally handled by supplemental approvals. And with its pivotal trial being relatively small and involving direct comparison with another recombinant Factor VIII product and with the CMC portion of the Xynta BLA surely citing and/or repeating that from ReFacto's BLA, this approval has characteristics of a follow-on biologic/biosimilar-type approval. Xyntha will largely inherit the market of its predecessor, ReFacto, with $335 million sales in 2007.
12) Accretropin, recombinant somatropin (human growth hormone), from Cangene with U.S. marketing by Apotex, joins multiple other E. coli-expressed somatropin products. Receiving 505(b)(2) generic drug approval based largely on comparisons with Humatrope from Eli Lilly, Accretropin offers no substantive safety or effficacy advantages relative to comparable products; and could be considered a follow-on protein- or biosimilar-type approval. However, Accretropin is not identical, substitutable/interchangeable or a biogeneric equivalent of Humatrope, and is considered a new, unique/distinct product.
13) Recothrom, recombinant human thrombin, from ZymoGenetics, Inc. received approval as a clotting agent (similar to fibrin glue) to help halt bleeding from small blood vessels after surgery. Previously, only bovine (cow)-derived thrombin, Thrombin-JMI, was available in the U.S. Recombinant thrombin provides decreased theoretical risk of exposure to non-human prions and viruses, and apparently has fewer side effects, but will likely not significantly change patient treatment or outcomes. Although offering less risk for side effects compared to Thrombin-JMI, ZymoGenetics has been chastised by FDA for making such claims (with Recothrom's trials not explicitly designed to test for this). With Thrombin-JMI well-established in the U.S. market and U.S. market uptake of Recothrom relatively slow so far, its largest near-term market may be in Europe where Thrombin-JMI is not available. Peak sales of Recothrom are projected to be in the $300-$500 million/year range.
What About the Future?
Biopharmaceutical approvals should increase steadily in coming years, with 2008 likely (or hopefully) becoming a recent low point. A large number (>25) filings are currently pending and an equally large number are expected to be filed in 2009, so there should be an increase in new products in 2009 and the next few years. Pending and expected filings include a number having previously experiencing setbacks/problems (presumably fixed) with their clinical trials and other parts of their applications. The pending and expected filings include a number of recombinant monoclonal antibodies currently in later stages of development. And within the next few years, a number of biosimilars/follow-on proteins/biogenerics will enter the market. Many of these, despite containing much the same or similar active agents as prior products, will be substantially innovative and offer significant improvements, such as novel methods for administration. And along with these, in the next few years there will be many filings involving new classes of marketed products, including gene therapies, cancer vaccines, stem and other cellular therapies, cultured tissue transplants and individualized biopharmaceuticals, with many of these products (notably gene therapies) long in development, along with newer technologies, e.g., RNAi.
However, various factors could combine to restict future biopharmaceutical product approvals to their recent low levels. With large numbers of applications filed and expected to be filed in 2009, it is easy and tempting to predict a significant increase in approvals in 2009 and coming years. Yet, prior predictions by this and other analysts of an increase in biopharmaceutical approvals were not born out in 2008. If problems with company filings and delays and rejections by FDA worsen, which this analyst considers unlikely, low levels of FDA biopharmaceutical approvals will continue in coming years. No matter what happens, more products will be available in the future. However, along with a number of truly innovative and novel products/agents, besides biogeneric/biosimilars/follow-on biologics entering the U.S. market in coming years, there will also be additional me-too-type (competing for the same indications) and derivative products, resulting in more competition for all products, including both well-established and innovative new products.
Conclusions
The biopharmaceutical industry, with the U.S. clearly the leader, is dependent on innovation -- more new products for new indications and new markets, which by necessity includes new blockbusters. Over $10 billion/year is being spent annually on biopharmaceutical R&D (conservatively presuming <20% of total pharmaceutical R&D involves biopharmaceuticals), but recent years' new products do not include blockbusters or even when combined do not attain this level. Everyone agrees that an increasing number and percentage of pharmaceuticals in development are biopharmaceuticals (vs. small molecule and other drugs). However, this has yet to be reflected in FDA approvals, with approvals of new biopharmaceutical products remaining at a low level and decreasing in recent years, with an increasing number and percentage experiencing delays and other problems in their late-stage development and FDA filings. If biopharmaceutical approvals, their novelty and heathcare and economic impact remain at recent low levels, the industry is ultimately headed for serious problems, potentially even economic collapse. The industry clearly needs more products with significantly more sales, particularly including blockbusters. However, with a large number of products pending approval, a large number of filings expected and with whole new classes of products completing their development, biopharmaceutical approvals and their markets should significantly increase in coming years.
|