FDA Biopharmaceutical Approvals Increased in 2009*
![]()
FDA Biopharmaceutical Approvals Increased in 2009*
![]()
This study was prepared by Mr. Ronald A. Rader, President, Biotechnology Information Institute, and author of BIOPHARMA: Biopharmaceutical Products in the U.S. and European Markets, the only information resource specializing in biopharmaceuticals. Mr. Rader remarked, "Overall, 2009 was a good year for FDA biopharmaceutical approvals, with 2009 approvals rising from the abysmally low levels of recent years to levels at least in line with approvals over the past decade. Biopharmaceutical approvals will further increase in coming years, with a large number of filings pending and many more expected, including for new classes of products, such as gene therapies, stem and other cellular therapies, and cancer vaccines."
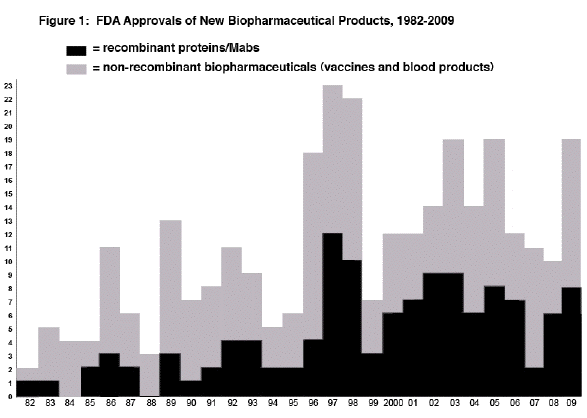
There were 18 full approvals for biopharmaceutical products in 2009. Eight of these were for recombinant proteins/antibodies, up from only 2 in 2007. All approvals involved products regulated by FDA as biologics, with these receiving BLAs, except for one (Creon) that received approval as a drug (NDA).
Among the 18 biopharmaceuticals receiving full approval in 2009, 16 are considered New Biopharmaceutical Entities (NBEs), i.e., are new, unique, distinct products. This is a significant increase, up from 10, 11 and 11 in 2008, 2007 and 2006, respectively. Unlike in recent years, there were no biogeneric/biosimilar-like [505(b)(2) generic drug] approvals.
No biopharmaceuticals received approval in 2009 for cancer indications, despite a large number reported to be in development. This continues a trend with none also approved in either 2008 or 2007. New biopharmaceuticals, including recombinant antibodies, for cancer treatment are widely extolled as on track to revolutionize patient care. So far, in terms of approvals, this is not the case.
Three recombinant monoclonal antibodies, all fully human, were approved in 2009, a significant increase from only one each in 2006-2008.
In terms of economic impact, peak sales for 2009-approved products are expected to cumulatively be greater than for products approved in 2008 and 2007. However, at best, only a few products may achieve blockbuster status (sales over $1 billion/year).
Many of these newly-approved products will significantly improve patient care. However, nearly all of these therapeutic advances are for orphan indications, so the number of patients affected and the economic impacts of these products will be somewhat limited.
2009 provided some notable technological advances. Cervarix became the first insect cell cultured recombinant biopharmaceutical and the first vaccine approved in the U.S. with a totally new adjuvant AS(04), composed of 3-deacylated monophosphoryl lipid A (MPL; derived from Salmonella minnesota). ATryn, recombinant antithrombin-III expressed in the milk of transgenic goats, became the first transgenic protein to receive approvals in the U.S. and European Union, a significant precedent for transgenic animal-derived therapeutic proteins.
The BIOPHARMA Web database reports at least 30 biopharmaceutical filings currently pending with FDA and at least 30 expected to be filed in 2010. Although it is too early to conclude that problems and delays in recent years with FDA reviews of biopharmaceutical filings have subsided and whether these were attributable to the FDA and/or sponsors, further increases are expected in 2010 and coming years.
The full study is online at www.biopharma.com/approvals_2009.html.
Figure 1. Biopharmaceutical approvals by year, starting in 1982, when the first recombinant protein received approval.
|
| ||||
| s vWF/Factor VIII Complex (Wilate) | Octapharma USA, Inc. | 12/4/2009 | von Willebrand's disease** | |
| Kallikrein inhibitor, rDNA (Kalbitor) | Dyax Inc. | 12/1/2009 | hereditary angioedema (HAE)** | |
| Influenza vaccine/Novartis Italy (Agriflu) | Novartis | 11/27/2009 | influenza prophylaxis | |
| CD20 Mab, human, rDNA (Arzerra) | GlaxoSmithKline (and Genmab) | 10/26/2009 | chronic lymphocytic leukaemia** | |
| Antitrypsin, alpha-1, conc./Talecris (Prolastin-C) | Talecris Biotherapeutics | 10/19.2009 | alpha1-antitrypsin (AAT) deficiency** | |
| HPV vaccine, rDNA/GSK (Cervarix) | GlaxoSmithKline | 10/16/2009 | cervical cancer prophylaxis | |
| C1-esterase inhibitor/CSL (Berinert P) | CSL Behring LLC | 10/09/2009 | hereditary angioedema (HAE)** | |
| IL-12/23 p40 Mab, rDNA (Stelara) | Ortho Biotech Inc. (Johnson & Johnson) | 9/25/2009 | plaque psoriasis** | |
| Immune Globulin (IGIV)/Bio Products (Gammaplex) | Bio Products Lab. | 9/17/2009 | primary immunodeficiency** | |
| Haemophilus b Vaccine/GSK (Hiberex) | GlaxoSmithKline | 8/19/2009 | Haemophilus influenzae prophylaxis | |
| Interferon betaser, rDNA/Novartis (Extavia)* | Novartis (and Bayer Schering) | 8/15/2009 | multiple sclerosis** | |
| Interleukin-1 Mab, rDNA (Ilaris) | Novartis | 6/17/2009 | Cryopyrin Associated Periodic Syndrome** | |
| Pancreatic Enzyme/Solvay (Creon)* | Solvay | 5/1/2009 | pancreatic enzyme insufficiency | |
| Botulinum Toxin A/Ipsen (Dysport; Reloxin) | Ipsen | 4/29/2009 | cervical dystonia**; gabellar (frown) lines | |
| TNF Mab, rDNA, human/J&J (Simponi) | Centocor Ortho Biotech Inc. (Johnson & Johnson) | 4/24/2009 | immune dysfunction-related arthritis | |
| Japanese Encephalitis Vaccine/Intercell (Ixiaro) | Intercell Biomedical (and Novartis) | 3/30/2009 | Japanese encephalitis virus prophylaxis | |
| Antithrombin III, rDNA (ATryn) | GTC Biotherapeutics, Inc. (Genzyme) | 2/6/2009 | antithrombin deficiency** | |
| Fibrinogen/CSL (RiaSTAT) | CSL Behring | 1/16/2009 | congenital fibrinogen deficiency** | |
![]()
Contact: