FDA Biopharmaceutical Product Approvals and Trends: 2010
![]()
FDA Biopharmaceutical Product Approvals and Trends: 2010
![]()
Background - Data are derived from the BIOPHARMA: Biopharmaceutical Products in the U.S. and European Markets Web database, the only reference/information resource specializing in biopharmaceuticals, with the product listings below linked to free public (partial) records from the database. See also the running list of recent years' approvals. Earlier analyses include 2009; 1982-2008, a related article from Gen. Eng. News and 2008 approvals.
Basic Data/Graphs - Figure 1 shows the number of approvals of new biopharmaceutical products by year since 1982 (when the first recombinant protein was approved).
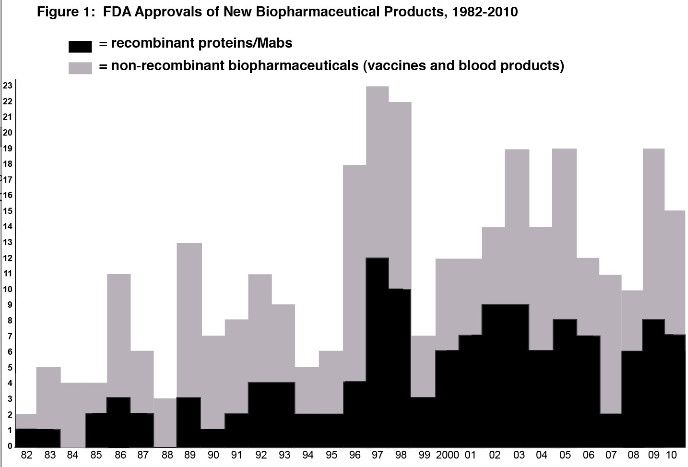
|
|
||||
| Urate oxidase, rDNA, PEG-* | Sanofi Pasteur | 9/14/2010 | chronic refractory gout | |
| Botulinum Toxin A/Merz | Merz Pharmaceuticals | 8/2/2010 | cervical dystonia; blepharospasm | |
| Antitrypsin, alpha-1/Kamada | Kamada Ltd. | 7/1/2010 | alpha1-antitrypsin deficiency | |
| RANKL Mab, rDNA | Amgen Inc. | 6/1/2010 | osteoporosis | |
| Glucosidase, rDNA/Lumizyme* | Genzyme Corp. | 5/25/2010 | Pompe disease | |
| Prostate Cancer Cellular Vaccine (rDNA) | Dendreon Corp. | 4/29/2010 | metastatic prostate cancer | |
| Pancreatic Enzymes/J&J | Johnson & Johnson (J&J) | 4/12/2010 | pancreatic insufficiency | |
| Fibrin Sealant/TachoSil | Nycomed Austria GmbH | 4/2/2010 | hemostasis in cardiovascular surgery | |
| Immune globulin (SCIG) | CSL Behring | 3/4/2010 | primary immunodeficiency | |
| Glucocerebrosidase, rDNA/Shire* | Shire Pharmaceutical | 2/26/2010 | Gaucher Disease | |
| Pneumococcal Vaccine(13)-CRM197 | Pfizer | 2/24/2010 | Streptococcus pnuemoniae-related disease | |
| Meningococcal Conjugates Vaccine/Novartis | Novartis | 2/19/2010 | meningococcal disease prevention | |
| Collagenase | Auxilium Pharmaceuticals Inc. | 2/3/2010 | Dupuytren's disease | |
| Glucagon-like peptide-1, rDNA | Novo Nordisk | 1/25/2010 | type 2 diabetes | |
| Interleukin-6 receptor Mab, rDNA | Amgen | 1/8/2010 | rheumatoid arthritis | |
More New Product Approvals
There were 15 full approvals (BLAs, NDAs) for biopharmaceutical products in 2009. Seven of the 15 approvals were for recombinant proteins, including 2 monoclonal antibodies. All approvals except 3 (Collagenase; Glucocerebrosidase, rDNA/Shire; Pancrelipase/J&J; Glucosidase, rDNA/Lumizyme) involved products regulated by FDA as biologics, with these 4 products receiving NDAs.
The BIOPHARMA: Biopharmaceutical Products in the U.S. and European Markets Web database reports 57 product applications currently pending with FDA or expected to be filed in 2010 (on 2/15/11).
Among the 18 biopharmaceuticals receiving full approval in 2009, 16 are considered New Biopharmaceutical Entities (NBEs), i.e., are new, unique, distinct products. This is a good number, up from 10, 11 and 11 in 2008, 2007 and 2006, respectively, but less than the 16 approved in 2009.
In 2010, as in 2009, there were no approvals of biogeneric-like biopharmaceuticals, such as 505(b)(2) generic drug approvals.
[Like most FDA approval analyses, biologics formally receiving full BLAs, such as Plasma and Red Blood Cells, but manufactured by local and regional blood centers have been excluded]. .
Medical Advances and Economic Impact
Two recombinant monoclonal antibodies were approved in 2010. The large number of chimeric, humanized, fully and de novo-designed human monoclonal antibodies that many companies and much of the industry are relying on for new products and continued survival are just starting to enter the market, with further increases likely in coming years.
Only one biopharmaceuticals, Provenge [Prostate Cancer Cellular Vaccine (rDNA)], received approval in 2010 for cancer indications, despite a large number of these in development. This broke the trend with none approved in either 2009, 2008 or 2007. New biopharmaceuticals, including recombinant antibodies, for cancer treatment are widely hyped as expected to revolutionize patient care, and provide industry with much-needed revenue. So far, in terms of approvals, this has yet to materialize.
Companies and Countries as Sources
No company received more than one approval in 2010. All 2010 products are being manufactured in the U.S., except for alpha-1-Proteinase Inhibitor (Human) [Glassia], manufactured in Israel, and Fibrin Sealant/TachoSil, manufactured in Austria.
What About the Future?
FDA biopharmaceutical approvals should further increase in coming years. At least 30 applications are pending with FDA and at least another 30 are expected to be filed in 2010 (see the BIOPHARMA database). Also In coming years, a number of biosimilars and biobetters will enter the U.S. market, with many new companies worldwide entering the U.S. biopharmaceutical marekt. Many of these products, despite containing much the same or similar active agents as prior products, will be substantially innovative and offer significant improvements, such as novel methods for administration. Also in the next few years, there will be many filings involving new classes of marketed products, including gene therapies, cancer vaccines, stem and other cellular therapies, cultured tissue transplants and individualized biopharmaceuticals, with many of these products (e.g., gene therapies) in development for decades, along with newer technologies, such as RNAi.
|