FDA Biopharmaceutical Product Approvals and Trends: 2011
![]()
FDA Biopharmaceutical Product Approvals and Trends: 2011
![]()
by Ronald A. Rader, President
Background - Data are derived from the BIOPHARMA: Biopharmaceutical Products in the U.S. and European Markets Web database, the only information resource/reference specializing in biopharmaceuticals.
See also the running list of recent years' approvals.
[Earlier analyses include
2010;
2009;
2008;
1982-2008 analysis, and
a related article from
Gen. Eng. News].
Biotechnology Information Institute
These are the only analyses of FDA biopharmaceutical approvals using fully consistent criteria from year-to-year.
'Biopharmaceuticals' are pharmaceuticals with therapeutic indications
manufactured using living organisms (biotechnology). This excludes 'drugs,' i.e.,
pharmaceuticals manufactured by chemical means, including synthetic drugs and
and natural products derived from dead tissues (e.g., heparins)
[1,2].
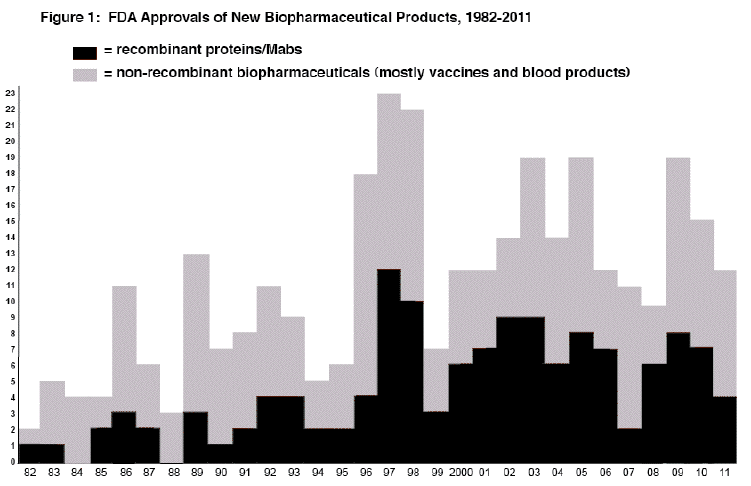
Figure 1 shows the number of full approvals of new biopharmaceutical products by year since 1982 (when the first recombinant protein was approved). Table 1 lists the product approvals.
|
|
||||
| VEGF Trap, rDNA (Eylea) | Regeneron Pharmaceuticals Inc. | 11/18/2011 | age-related macular degeneration | |
| Asparaginase/Erwinia (Erwinaze)* | EUSA Pharma Inc. | 11/18/2011 | acute lymphoblastic leukemia | |
| Cord blood stem cells (Hemacord) | New York Blood Center, Inc. | 11/10/2011 | hematopoietic stem cell transplantation | |
| CD30 mAb, rDNA--monomethyl auristatin E (Adcetris)* | Seattle Genetics, Inc | 8/19/2011 | Hodgkin's lymphoma | |
| Scorpion antitoxin/immunoglobulin, equine (Anascorp)* | Rare Disease Therapeutics Inc. | 8/3/2011 | scorpion stings | |
| Fibroblasts, autologous (laViv)* | Fibrocell Science, Inc. | 6/22/2011 | nasolabial fold wrinkles (smile lines) | |
| CTLA4-Ig, rDNA (Nulojix)* | Bristol-Myers Squibb | 6/15/2011 | kidney transplant rejection | |
| Albumin, human (Kedbumin)* | Kedrion, S.p.A. | 6/3/2011 | multiple albumin supplementation indications | |
| CTLA-4 Mab, rDNA/Medarex (Yervoy)* | Bristol-Myers Squibb | 3/25/2011 | melanoma | |
| Adenovirus Type 4, 7 Vaccine Live, oral (Ardovax) | Teva Pharmaceuticals | 3/16/2011 | vaccination of U.S. military personnel only | |
| B-cell-activating factor Mab, rDNA (Benlysta) | Human Genome Sciences Inc. | 3/9/2011 | systemic lupus erythematosus | |
| Factor XIII, human (Corifact)* | CSL Behring | 2/17/2011 | Factor XIII deficiency | |
There were 12 full approvals for biopharmaceutical products in 2011. All of the qualifying products were approved as biologics (BLAs), except for 1 drug (NDA; Erwinaze, grandfathered as a drug). Only 4 of the 12 (33%) approvals were for recombinant proteins, a low number and percentage. This included 3 monoclonal antibody-based products.
Medical Advances/Novel Indications: A record number of biopharmaceuticals received approval for novel indications (diseases), including for indications for which no prior effective treatments were available and for indications for which there had been no new approvals for over a decade. But various approvals are more specific and limited than can be so simply described (Table 1), e.g., not for first-line use; and some of these products' sales are already much less than had been expected (e.g., Benlysta). We may be starting to see prior increased industry emphasis on orphan development finally coming to fruitiion in terms of product approvals. Treatment advances and innovations include:
Two products received approval for a cancer indications, following 1 in 2010 and none in 2007, 2008 or 2009 (3 in the past 5 years). This is despite a large number of cancer-targeting biopharmaceuticals, particularly monoclonal antibodies, reported to be in development and often hyped as expected to revolutionize patient care and provide the industry with much-needed revenue, including new blockbusters. However, growth in the number of cancer-targeted biopharmaceutical approvals has yet to materialize as a distinct trend.
Some of these products are not new in terms of availability and use in the U.S. (further limiting the impact of 2011 approvals). Anascorp has been available for years for emergency use, including supply by CDC; Erwinaze (as Erwinase) has been available for decades for patients allergic to the prior E. coli-derived enzyme, including supply by NCI/NIH; the adenovirus vaccines (considered to be a single combination vaccine) are replacements for vaccines that the Dept. of Defense (DOD) had previously purchased, but had neglectfully (in hindsight) let their manufacture and approvals lapse; and the equivalents of Hemacord (stem cells transplantation) and IaViv (patient's own fibroblasts), like other individualized blood processing-based treatments, have been available to patients through custom processing rather than as approved products (with approvals supporting consistent manufacture, marketing and insurance coverage). And the adenovirus vaccine is only approved for DOD inoculation of new recruits entering boot camp.
Medical Advances/Orphan Approvals: A record number (8) and percentage (66%) of all 2011 approval, received approval with orphan designation (granting 7 years of market exclusivity). These are shown in Table 1. Although these products are major advances for affected patients and the nation's health care system, the markets for most of these are inherently limited, including many approvals being other than for first-line use and more limited than can be briefly described (in Table 1)
Active Agent Novelty:
Among the 12 biopharmaceuticals receiving full approval in 2009, all but one, a high purity human-derived albumin product (Kedbumin), are considered New Biopharmaceutical Entities (NBEs), i.e., are new, unique, distinct products, particularly in terms of molecular structure, composition or source. Such high percentages of NBEs, indicative of innovation, among biopharmaceutical approvals is not unusual. Precedents in terms of novel active agent approvals include:
A record number (5) of recombinant antibody, antibody-like or antibody portion-containing products received approval (including Nulojix and Eylea, a VEGF "trap" molecule).
We may finally be starting to see industry's considerable investments on the development of recombinant antibody and antibody-like products paying off in terms of products entering the market.
Another product, Anascorp, involves human plasma-dervied antibodies.
Other Findings:
Economic and Industry Impacts:
2011 was a poor year in terms of the projected economic impact of approved products, with a low number of approvals and the sales of the many approved products with orphan or other limited, e.g., not first-line or other mainstream, indications providing only small niche markets. Yervoy and Benlysta have been the only approved products cited as potential blockbusters (>$1 billion/year), and Nulogix has been reported to potentially attain sales of over $500 million/year. But already, sales of some have been considerably well below expectations, e.g., Benlysta had only $52 million in sales after nearly 9 months on the market, while Yervoy, which was approved later, had about $350 million sales in 2011. The other 9 products approved will attain much smaller markets.
The number of biopharmaceuticals approved and their economic impact need to increase to assure continued vitality of the (bio)pharmaceutical industry. It is now reported (e.g., by PhRMA and Burrill & Co.) that pharmaceutical R&D is about $70 billion, a record high, with likely about 40% ($27 billion) of this now involving biopharmaceuticals. More biopharmaceuticals with higher sales are needed to cover the costs of the record amounts being invested in biopharmaceutical R&D. This includes many of the largest international (Big Pharma) companies now investing nearly half or even more of their R&D in biopharmaceuticals (vs. drugs), with much of this going for development of monoclonal antibodies and cancer therapeutics. However, despite years of investment and hype, relatively few of these products are yet entering the market.
The reasons for low biopharmaceutical approvals remain unclear.
It is easy to blame increased scrutiny and higher standards on the part of FDA, but there are no data to support this (with FDA handling of filings far from transparent).
Recent findings from the BIO/BioMedTracker study of pharmaceutical approval rates indicate that products regulated as biologics have a relatively high overall success rate, 26%, i.e., 26% of biologics that enter trials eventually receive FDA approval for their primary/lead indication(s). This compares to 14% for New Molecular Entity (NME; fully novel) products regulated as drugs.
So FDA does not appear to be holding biologics to significantly more difficult-to-attain standards compared to drugs.
One can also cite the economic difficulties in recent years, which surely have restricted R&D budgets and likey reduced the number of products entering late-stage trials. Continued merging and purging among many of the largest pharmaceutical companies continues to result in R&D pipeline shrinkage.
Besides there simply being fewer major players, mergers of large companies often result in their R&D pipelines being trimmed back to much the same or fewer projects than the acquiring company started with.
Starting in a few years, we can expect a significant increase in biopharmaceutical approvals. Even ignoring innovative products, this includes multiple biosimilar (approved through the new biosimilars pathway) and biobetter (follow-ons receiving full approval) products entering the market for just about most every currently successful product coming off patent in coming years. Thus, the number of recombinant proteins (130+) marketed in the U.S. and the number of companies involved could double in the next 5 years. Biosimilars (and biobetters and biogenerics) will bring more competition and lower prices, but these inherently me-to products will also result in market fragmentation; will not expand the biopharmaceutical market; do not provide new treatments, including for new indications; and with increased competition, sales levels and profit margins will be lower than with innovative products. Thus, commercialization of biosimilars (and biobetters and biogenerics) as current products go off-patent will expand the number of products and players, but not significantly advance the economic health of the (bio)pharmaceutical industry.
But in the next few years, there will likely be filings involving newer classes of products, such as gene therapies, cancer vaccines, stem and other cellular therapies, RNAi, cultured tissue transplants and individualized biopharmaceuticals.
Industry and Country Developments
Some developments and findings related to companies and countries included:
Conclusions:
2011 was a good year for patient and health care advances, particularly biopharmaceutical approvals for some new and orphan indications. However, 2011 was poor in terms of the number of approvals and their projected economic impact.
With many of the largest (bio)pharmaceutical companies, the major players, counting on biopharmaceuticals to provide their future profits, if the low number of approvals and their economic impact persist, the industry is headed for big trouble.
|