FDA Biopharmaceutical Product Approvals and Trends: 2012
![]()
FDA Biopharmaceutical Product Approvals and Trends: 2012
![]()
by Ronald A. Rader, President
Background - These findings are derived from the BIOPHARMA: Biopharmaceutical Products in the U.S. and European Markets Web database, the only information resource/reference specializing in marketed biopharmaceutical products.
See also the running List of FDA biopharmaceutical approvals.
These annual analyses of FDA biopharmaceutical approvals are the only ones using consistent criteria from year-to-year for what is (and is not) a new and unique biopharmaceutical, and/or do not arbitrarily include small molecule drugs.
'Biopharmaceuticals' are determined using an agent/product-centric approach, i.e., the type of approval received (e.g., full vs. supplemental, BLA or NDA) is not the primary determinant.
'Biopharmaceuticals' refers to pharmaceuticals with therapeutic indications
manufactured using living organisms (biotechnology). This excludes 'drugs,' i.e.,
pharmaceuticals manufactured by chemical means, including synthetic drugs and
natural products derived from dead tissues (e.g., heparins)
[1,2].
Biotechnology Information Institute
The overall health care impact and projected peak sales of 2012-approved products are rather limited. Many of the approved products, perhaps a majority, are biobetter, me-too or similar follow-on products, and many are orphan products, with these types of products inherently limited in terms of their innovation, novelty and/or markets. The approved products do not include any likely blockbusters (>$1 billion/year); and most products appear unlikely to even attain average biopharmaceutical sales levels.
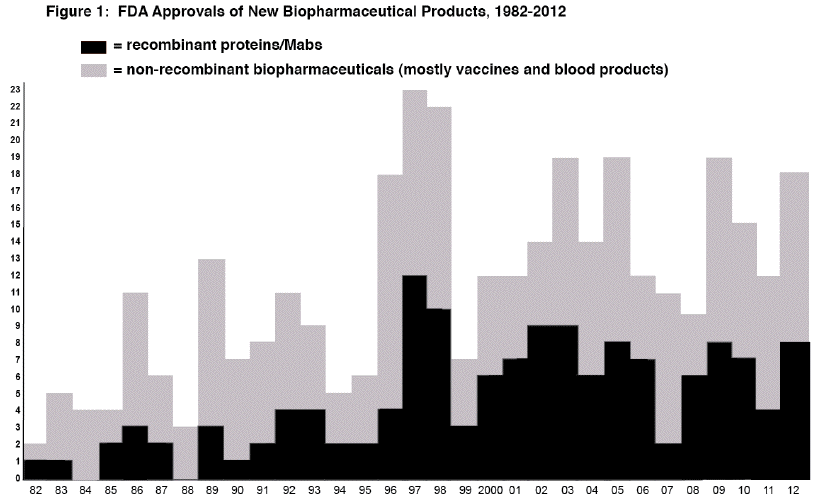
2012 FDA biopharmaceutical approvals were numerically inadequate, in the sense that there is still no trend for an increase in approvals (further discussed below). It is now commonly reported that an increasng percentage, now over 40%, of all pharmaceutical industry R&D and products in the pipeline involve biopharmceuticals rather than drugs. However, this has yet to be refected at all in FDA approvals, with approvals relatively flat, still with no discernable trend for increase since the later 1990s.
Records set in 2012 include record numbers and percentages of:
1) lesser innovative/novel biobetter me-too-type follow-on products
2) products approved with orphan status
3) products manufactured outside of the U.S., including the first Eastern European-manufactured product
4) products involving the largest Big Pharma-type companies.
None of these records yet exemplify trends. But these records set in 2012 do not reflect or bode well for significantly increased biopharmaceutical industry innovation; industry market expansion; smaller biotechnology company-based (vs. Big Pharma) success in biopharmceuticals; or expansion of the U.S.-based biopharmaceutical industry.
Figure 1 shows the number of approvals of new biopharmaceutical products by year since 1982 (when the first recombinant protein was approved).
Table 1 below presents information about each of the 2012 approvals.
|
|
||||
| glucagon-like peptide 2, rDNA (Gattex)** | NPS Pharmaceutical | 12/21/2012 | short bowel syndrome (SBS) | |
| Immune Globulin (IGIV)/Biotest (Bivigam) | Biotest Pharmaceuticals Corp. | 12/20/2012 | primary immune deficiency disorders (PIDD) | |
| Varicella-Zoster Immune Globulin/Cangene (VariZIG) | Cangene Corp. | 12/19/2011 | prophylaxis of varicella | |
| Influenza Vaccine, quadravalent (Fluarix Quadravalent)* | GlaxoSmithKline (GSK) | 12/19/2011 | seasonal influenza vacccine | |
| Anthrax Mab, rDNA (ABthrax) | Human Genome Sciences Inc., GlaxoSmithKline (GSK) | 12/14/2012 | treatment and prevention of inhalational anthrax | |
| Fibrin Sealant Patch/J&J (Evarrest) | Ethicon Biosurgery, Johnson & Johnson (J&J); Omrix Biopharmaceuticals Ltd. | 12/7/2012 | stop bleeding during surgery | |
| Influenza vaccine, MDCK cultured (Flucelvax) | Novartis | 11/20/2012 | seasonal influenza vacccine | |
| Microplasmin, rDNA (Jetrea) | ThromboGenics | 10/18/2012 | vitreomacular adhesion | |
| G-CSF, rDNA/Teva (Neutroval) | Sicor Biotech (Teva Pharmaceuticals) | 8/30/2012 | neutropenia associated with cancer treatment | |
| VEGF Trap, rDNA (Zaltrap) | Regeneron Pharmaceuticals; Sanofi S.A. | 8/3/2012 | refractory metastatic colorectal cancer | |
| MenC-Hib vaccine (MenHibrix) | GlaxoSmithKline (GSK) | 6/14/2012 | Neisseria meningitidis and Haemophilus influenzae vaccine | |
| HER2 receptor Mab, rDNA (Perjeta) | Genentech/Roche | 6/8/2012 | breast cancer, combination use | |
| Glucocerebrosidase, rDNA (Elelyso)** | Protalix BioTherapeutics Inc.; Pfizer | 5/1/2012 | Gaucher disease | |
| Human cells, autologous/bovine collagen substrate (Gingtuit) | Organogenesis Inc. | 3/9/2012 | oral tissue surgical wound repair | |
| Pancreatic enzymes (Ultresa)** | Aptalis Pharma | 3/1/2012 | pancreatic insufficiency | |
| Pancreatic enzymes (Viokace)** | Aptalis Pharma | 3/1/2012 | pancreatic insufficiency | |
| Influenza vaccine, live intranasal quadravalent (FluMist Quadrivalent)* | MedImmune (AstraZeneca) | 2/29/2012 | seasonal influenza vaccine | |
| Carboxypeptidase, rDNA (Voraxaze) | BTG plc (formerly Protherics) | 1/18/2012 | methotrexate toxicity treatment | |
New Biophamaceutical Entities (NBEs): Nine (50%) newly-approved products are considered New Biophamaceutical Entitities (NBEs), i.e., substantially novel and innovative products (based on active agent structures and/or bioprocessing, compared to those with prior FDA approval):
This is a rather low and disappointing outcome for the (bio)pharmaceutical industry, particularly, considering that
a) an increasing number of companies, including an increasing number of the largest companies, are increasingly dependent on new innovative biopharmaceuticals for their future survival;
b) these NBEs collectively do not reach projected blockbuster levels (>$1 billion/year revenue; see market impact discussion below); and
c) the degree of innovation/novelty exemplified in these products varies greatly
These 9 NBEs are 50% of 2012 new biopharmaceutical approvals, compared to 12 NBEs in 2011, 92% of the total. So both the number and percentage of new innovative/novel products were significantly lower in 2012. Last year, all but one approval represented a significant advance for patients and the health care system, mostly new products for new indications, but this was far from the case with 2012 approvals.
Also, close examination of 2012-approved products illustrates the continuous spectrum of innovation/novelty exemplified in biopharmaceutical products. Many of the 2012 NBEs may be considered borderline, depending on criteria used. If one were to score the relative innovativeness/novelty of these 2012 NBE approvals, rather than judging them as either NBE or not, the degree of innovation and novelty for many would be on the low side. For example, the two quadrivalent influenza vaccines receiving supplemental approvals simply involve addition of a similar 4th antigen, and even the cancer monoclonal antibody, Perjeta, could be considered a trivial follow-on or biobetter version of another already-marketed product from the same company. The only products that can be considered fully innovative and novel, i.e., nothing rather similar already approved, are Gattex (glucagon-like peptide 2, rDNA), Voraxaze (carboxypeptidase, rDNA), Jetrea (microplasmin, rDNA) and ABthrax (anthrax mAb, rDNA). The other NBEs are innovative/novel due to their bioprocessing.
Only 3 recombinant monoclonal antibody or antibody-like products received approval in 2012, compared to 5 last year. Only one 2012 approval, a monoclonal antibody, has a cancer indication. There has been much hype about the large number, 100s, of monoclonal antibody products, particularly with cancer indications, in the development pipeline. But these have yet to result in approvals.
Lesser Innovative/Novel (Biobetter/Me-too) Follow-on Products: A total of 9 'biobetters' (50%) received approval in 2012. 'Biobetters' refers to follow-on products containing active agents that are substantially (bio)similar to those of prior-approved products, but the products are different enough from the products they follow-on such that they do not receive biosimilar or generic drug approvals. Thus, biobetters generally receive full approvals, the same as fully-innovative products. Biobetter/follow-on/me-too products containing rather familiar, not-all-that-new, active agents approved in 2012 were:
Orphan Products: A record number (9) and percentage (50%) were approved with orphan designation, granting these 7 years of market exclusivity (non-approval of substantially identical products for the same indications):
These orphan products are definitely welcome by patients needing them, but by definition, orphan products have limited market potential due to a limited number of treatable patients, with significant revenue only attainable by charging high prices. For example, Gattex, with a potential market of 3,000 patients, costs $295,000/year, and Elelyso also costs over $200,000/year.
Medical Advances/Novel Indications: None of the 2012 approvals stand out as breakthrough products having broad impacts for patients, e.g., approvals for new indications meeting major previously unmet needs. This is unlike last year, when nearly every approval involved indications for which no prior effective treatments were available or for indications for which there had been no new approvals for over a decade. [Keep in mind that the "Indications" reported above in Table 1 are much shortened and simplified, not the full official indications which generally include restrictions on elegible patients and product use]. Similarly, 2012 approvals will not result in significant increases in competition potentially leading to price decreases.. The more significant advances and innovations in terms of indications and patient treatment were:
Market and Health Care System Impacts: 2012 was another poor year in terms of health care system and public health impacts, with most products involving relatively small markets. Even the products embodying the most innovation and novelty are relatively limited in their real-world impacts, mostly for orphan or other indications affecting limited numbers of patients.. In the big picture, none of the approved products will save the health care system significant revenue; nor will any of these products significantly improve the nation's overall public health.
Overall, 2012 FDA-approved biopharmaceuticals will have little impact on worldwide annual biopharmaceutical revenue, now >$150 billion total and over $100 billion for recombinant proteins/antibodies. There are no potential blockbusters (>$1 billion/year), which the industry direly needs.. In fact, few of the 2012 approved products appear likely to attain even average peak sales, with the average annual revenue for biopharmaceuticals (marketed in the U.S. and/or EU) about $250-$300 million/year.
Microplasmin (Jetrea) has been projected to have a potential peak market of about $400 million. Some likely overly-optimistic projections foresee a market double this size, but these apparently include adoption for indications not explicitly covered by its initial approval. Gattex is projected by its developers's CEO as likely attaining $350 million/year in peak sales. Glucocerebrosidase (Elelyso), priced lower than its competition, could potentially capture a significant portion of the current ~$1 billion/year market for glucocerebrosidase products. The anthrax mAb (ABthrax), developed primarily for the U.S. biodefense stockpile, could potentially become an ~$100 million/year product, if one or more anthrax biological warfare attacks occur, terrorists acquire these weapons, etc., such that governments worldwide are forced to purchase this for their biodefense stockpiles. The other products appear unlikely to surpass even a few $100s millions in peak annual sales for their currently-approved indications.
Even all 2012-approved products together appear unlikely to attain eventual total peak sales (based on their current approved indications) comparable to that of a few blockbuster products (>$1 billion/year), the types of approvals with obvious broad impacts that the industry, patients and the health care system need most. Even if 2012 approvals eventually somehow add say $2.5-3.0 billion to biopharmaceutical sales, that is relatively insignificant, representing only about 1.6%-2.0% market growth relative to the current ~$150 billion worldwide biopharmaceutical market (with the market surely larger, the impact smaller, by the time 2012 approved-products attain their peak sales). Worldwide biopharmaceutical revenue has long been rather consistently growing at about ≥15% annually, driven by both new products and expansion of markets for existing products. Annula revenue growth of at best 2% due to new products (vs. expansion of markets for already-markets products) is relatively trivial, and likely indicative of serious problems.
Two products received approval for cancer indications, following 2 in 2012, 1 in 2010 and none in 2007, 2008 or 2009 (5 in the past 6 years). This is despite 100s of cancer-targeting biopharmaceuticals, particularly monoclonal antibodies, reported to be in development, with these often hyped as expected to revolutionize patient care and provide the industry with much-needed revenue, including new blockbusters. However, growth in the number of cancer-targeted biopharmaceutical approvals has yet to materialize as a distinct trend.
Biosimilars: No biologics received FDA biosimilar approval in 2012, and no biosimilar applications are even pending yet, despite the law (BPCIA) authorizing biosimilar approvals having been passed well over 2 years ago. However, one product that received full BLA approval, G-CSF from Teva (Neutroval), has received biosimilar approval in the European Union where it is marketed as TevaGrastim. Some may consider this product to be a biosimilar in this context (but not from a U.S.-centric perspective). As in 2011, 2010 and 2009, there were no biogeneric-like biopharmaceutical approvals, i.e., no 505(b)(2) generic drug approvals of biopharmaceuticals (and with the BPCIA redefining 'biologics' to include all biopharmaceuticals, including those historically regulated as drugs, this may be the end of these approvals).
Bioprocessing Innovations: The product by far embodying the the most bioprocessing innovation is Elelyso, the first plant-expressed FDA-approved biopharmaceutical. Elelyso is manufactured by suspension cell culture of transformed carrot cells in single-use bioreactors. Elelyso and its bioprocessing was developed by Protalix BioTherapeutics (Israel) with worldwide marketing by Pfizer.
Manufacturing Source Countries: A record number (8) and percentage (44%) of products approved in 2012 are manufactured outside of the U.S.:
The prior record for approvals of foreign-manufactured biopharmaceuticals was last year with 4 products (33%). Five 2012 products are manufactured in the European Union. The Neutroval approval represents the first approval for an Eastern European biologics manufacturing facility.
Company-related Findings:
Prospects for Future Approvals: A rapid and major increase in biopharmaceutical approvals is expected in coming years, includinhg more diverse types of products, including biosimilars. The BIOPHARMA: Biopharmaceutical Products in the U.S. and European Markets Web database reports nearly 50 products with full applications currently pending with FDA or expected to be filed in 2013; a number in line with those reported in recent years. In the next few years, there will also be many filings involving novel classes of products, such as gene therapies, cancer vaccines, stem and other cellular therapies, RNAi, cultured tissue transplants and individualized biopharmaceuticals. Marketed biopharmaceuticals will become more diverse in terms of their underlying technologies.
Besides these innovative products, a large number of approvals in coming years, perhaps even a majority, will include multiple biosimilars (approved through the biosimilars pathway) and, to a lesser extent, biobetters (follow-ons receiving full approval) entering the market for just about most every currently successful biopharmaceutical as each comes off patent. A recent study of the biosimilars/biobetters pipeline (by this author, see www.biosimilarspipeline.com, left column) reports over 425 biosimilars and 360 biobetters known to be in development (with this not including many of the future major players, e.g., the largest Big Pharma, generic drug and biopharmaceutical companies, which have yet to disclose their biosimilar pipelines).
Much as the drug Singulair (asthma treatment from Merck) recently went generic with 10 products approved on the first day possible, 10-12 or even more biosimilars/biobetters can be expected to enter the U.S. market (as soon as patents and market and data exclusivities expire) for each successful biopharmaceutical. This includes the over 60 current FDA-approved recombinant protein/mAb products with revenue over $500 million/year, including 37 with >$1 billion/year revenue; with these products able to support multiple competing biosimilars/biobetters. For example, capturing just 10% of the current ~$2.5 billion market for insulin lispro (Humalog, a fast-acting insulin analog) provides a market of $250 million. If just an average of 10 follow-ons receive approval for each current blockbuster biopharmaceutical, that represents over 350 new product approvals. Thus, the number of recombinant proteins (140+) approved in the U.S. and the number of companies involved will likely more than double, perhaps triple or more, in the next 5 years. However, biosimilars and biobetters will not expand the market or greatly increase treatment options.
More follow-on products (biosimilars and biobetters) will bring more competition and lower prices. But these inherently me-too products will also result in market fragmentation and contraction, with more competition at lower prices but with no expansion of the market for each group of similar products. The combined market for reference products and their follow-ons will tend to contract as price competition affects an increasing number and percentage of products. These me-too products do not increase basic treatment options and inherently lack significant innovation and novelty. Thus, commercialization of biosimilars (and biobetters and biogenerics) as current products go off-patent will very significantly expand the number of biopharmaceutical products and players, result in much-welcome price reductions, but will not significantly advance the overall revenue, the economic health of the (bio)pharmaceutical industry, innovation, or the treatment options available to patients.
More Products/Approvals Are Needed: The number of biopharmaceuticals approved and their economic impact need to signficantly increase to assure continued vitality of the (bio)pharmaceutical industry. It is now commonly reported that pharmaceutical industry R&D is now well over $80 billion/year with >40% of this involving biopharmaceuticals. More biopharmaceuticals with higher sales are needed to cover the costs of the record amounts that have been and are being spent on biopharmaceutical R&D. This includes many of the largest international (Big Pharma) companies now investing nearly half or even most of their R&D in biopharmaceuticals (vs. drugs), with much of this going for development of monoclonal antibodies and cancer therapeutics. However, despite years of investment and hype, relatively few of these products and, particularly, few blockbusters or other products with significant projected sales are yet entering the market.
Why Are Approvals Lagging?: The reasons for low biopharmaceutical approvals continue to remain diverse and unclear. It is easy to blame increased scrutiny and higher standards on the part of FDA, but there are no real data to support this. FDA does not appear to be holding biologics to significantly more difficult-to-attain standards compared to drugs. One can cite the economic difficulties in recent years, which surely have restricted R&D budgets and reduced the number of products entering late-stage trials. Merging and purging among many of the largest (bio)pharmaceutical companies and their addiction to outsourcing continues to result in shrinkage of these companies' resources, capabilities, staff and R&D pipelines. Mergers/acquisitions rationalized as boosting merged company R&D pipelines seem to rarely result in this.
FDA's Views of 'Biopharmaceutical' Approvals: It should be noted that FDA issued a report, "FY 2012 Innovative Drug Approvals, with this reporting 10 (28%) 'biopharmaceuticals' (by this author's definition and most conventional use) among the 35 "innovative" product approvals reported. The report presents no useful analyses of biologics (biopharmaceutical) approvals.
It is very disheartening to see FDA follow the lead of PhRMA and Big Pharma companies, which in recent years have been laying the groundwork for rebranding of themselves and the entire 'pharmaceutical' industry as 'biopharmaceutical' through promotion of indiscriminate and uncritical swapping of use of 'biopharmaceutical' in place of 'pharmaceutical [3].' For example, PhRMA cites its members and the entire industry as now 'biopharmaceutuical' (not drug or pharmaceutical). PhRMA publications vaguely cite a mystical/magical transformation of the industry (never explained how or when) into one that is now thoroughly dominated by biotechnology (but in reality, not in terms of products in the pipeline or marketed, R&D targets or expenses, sales revenue or any other relevant parameter). The very first sentence in the report's "Introduction" cites "35 novel medicines developed by the biopharmaceutical industry," with 'biopharmaceutical' never defined, despite FDA never having used this term in this context before (as far as this author is aware). Partricularly now that the BPCIA (biosimilars act) has redefined 'biologics' to be in line with that of 'biopharmaceuticals' (i.e., involving living organisms/biotechnology in their manufacture, as used in this study and by most in the industry), FDA needs to be more mindful of its adopting of improper, never-defined, purely-PR-oriented hype and terminology. It is easy to understand why Big Pharma would want to rebrand itself and the entire pharmaceutical/drug industry as 'biopharmaceutical' (sounds better, sexier, newer, higher-tech, etc.), but why would FDA adopt this terminology?
Conclusions:
2012 FDA biopharmaceutical approvals were not all that significant in terms of innovation or novelty, including new products for new indications, nor in terms of market, industry and health care impacts. Projected biopharmaceutical market/revenue increases attributable to 2012 approvals are insufficient. Similarly, there were few significant advances in patient care and few significant positive impacts on the health care system. 2012 was a record year for less-than-fully-novel and -innovative products, particularly biobetters and other me-too-type follow-on products. 2012 saw a record number of these biopharmceutical approvals, along with a record number manufactured outside the U.S. The same as last year, it can be concluded that, with many of the largest (bio)pharmaceutical companies, the major players, along with a large number of smaller biotech-type companies, essentially the entire pharmaceutical industry, counting on biopharmaceuticals to provides future profits, if the low number of approvals and their economic impact persist, the industry is headed for big trouble.
|