FDA Approvals - Review of 1982-2007 Plus Update for 2008 and Listings from 2006-Present
Based on
Biopharmaceutical Products in the U.S. and European Markets
![]()
FDA Approvals - Review of 1982-2007 Plus Update for 2008 and Listings from 2006-Present
Based on
Biopharmaceutical Products in the U.S. and European Markets
![]()
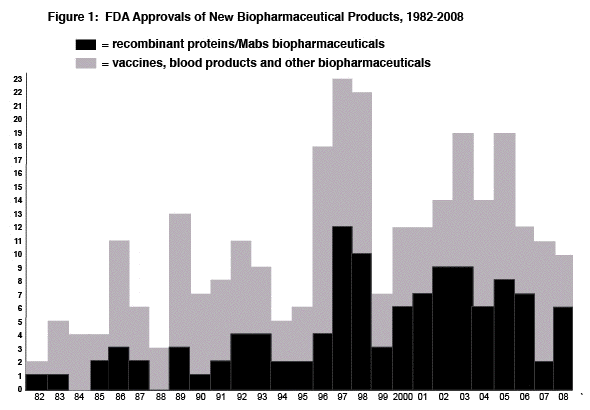
In the decade 1996-2005, there were an average of 16.6 approvals/year. The peak year was a decade ago, in 1997, with approval of 23 products including 12 recombinant proteins/Mabs. The numbers of new biopharmaceuticals approved in 2007 and 2006 were on the low side, just 11 and 10 respectively. 2007 was a particularly unproductive year. Most of the 2007 approvals were incremental advances, me-too products, and those with rather specialized indications. Besides a low level of novelty (with many products and their indications similar to those of prior products), none of the 2007 products are expected to significantly improve healthcare for large numbers of patients. The 2007 approval with by far the most potential impact, Mircera (pegylated recombinant erythropoietin; PEG-EPO), from Roche, will probably not be sold in the U.S. for at least several years due to patent infringement issues. Without Mircera, a likely blockbuster, the projected market for all products approved in 2007 does not even attain blockbuster level (>$1 billion/year revenue). The low number of recombinant protein/Mab approvals in 2007, only two, is more typical of the 1980s. And other than a formal approval for Epicel (cell cultured skin patches) from Genzyme, a product already marketed for 20 years, no established or mainstream U.S. biotechnology company received an approval, with approvals primarily granted to large foreign and small, new entrant, U.S. biotech companies. Hardly any Mabs or cancer therapeutics were approved in recent years, despite seemingly endless hype about large numbers in development. No biopharmaceuticals were approved for cancer indications in 2007, only one was approved in 2006, and only one Mab received approval in both 2006 and 2007. It is unclear why approvals have decreased and who, if anyone, deserves the blame for this. The low numbers of biopharmaceutical approvals in recent years are of particular concern, because a large number of products have been in the development pipeline. This includes hundreds of biopharmaceuticals that entered trials from the mid-1990s to mid-2000s, a period expected to result in approvals in recent years. For example, PhRMA reported 350 biotech medicines in development in 2000. Some analysts and company executives blame FDA for fewer approvals, asserting that recent controversies over the safety of various pharmaceuticals have resulted in FDA being overly cautious, defensive and unassertive, and note that FDA is taking a longer time and/or inducing delays in evaluating new products. However, Mr. Rader notes that relatively few filings for biopharmaceuticals have been arbitrarily delayed, put on long-term hold or denied. Rather, it appears that fewer products are successfully making it through pivotal Phase III trials. FDA may well be slowing down and shifting its approval criteria to be more restrictive, but most of the biopharmaceuticals affected by FDA delays or denials had problems, usually not attaining their preset primary endpoints in pivotal trials or otherwise having problems with safety or efficacy. It may be that inadequate staff and resources are taking their toll at FDA. Or, fewer approvals could well be the result of poor decisions, many made years ago, by industry sponsors. The reasons for declining approvals require further study and discussion. About two dozen or more filings for biopharmaceuticals are currently pending at FDA and about two dozen or more filings are expected in 2008. Thus, unless the great majority of pending and upcoming applications are rejected, delayed or abandoned, significant increases in approvals can be expected in 2008 and 2009. [Note, this prediction has yet to come to fruition]. The biopharmaceutical industry is dependent on innovoation -- new products for new indications and markets, which by necessity includes blockbusters. If biopharmaceutical approvals, their novelty and heathcare and economic impact remain at recent and, particularly, 2007 levels, the industry is headed for serious problems, potentially even economic collapse.
|